PermeaDerm® Biosynthetic Wound Matrix
Revolutionizing wound management for chronic wounds & burns
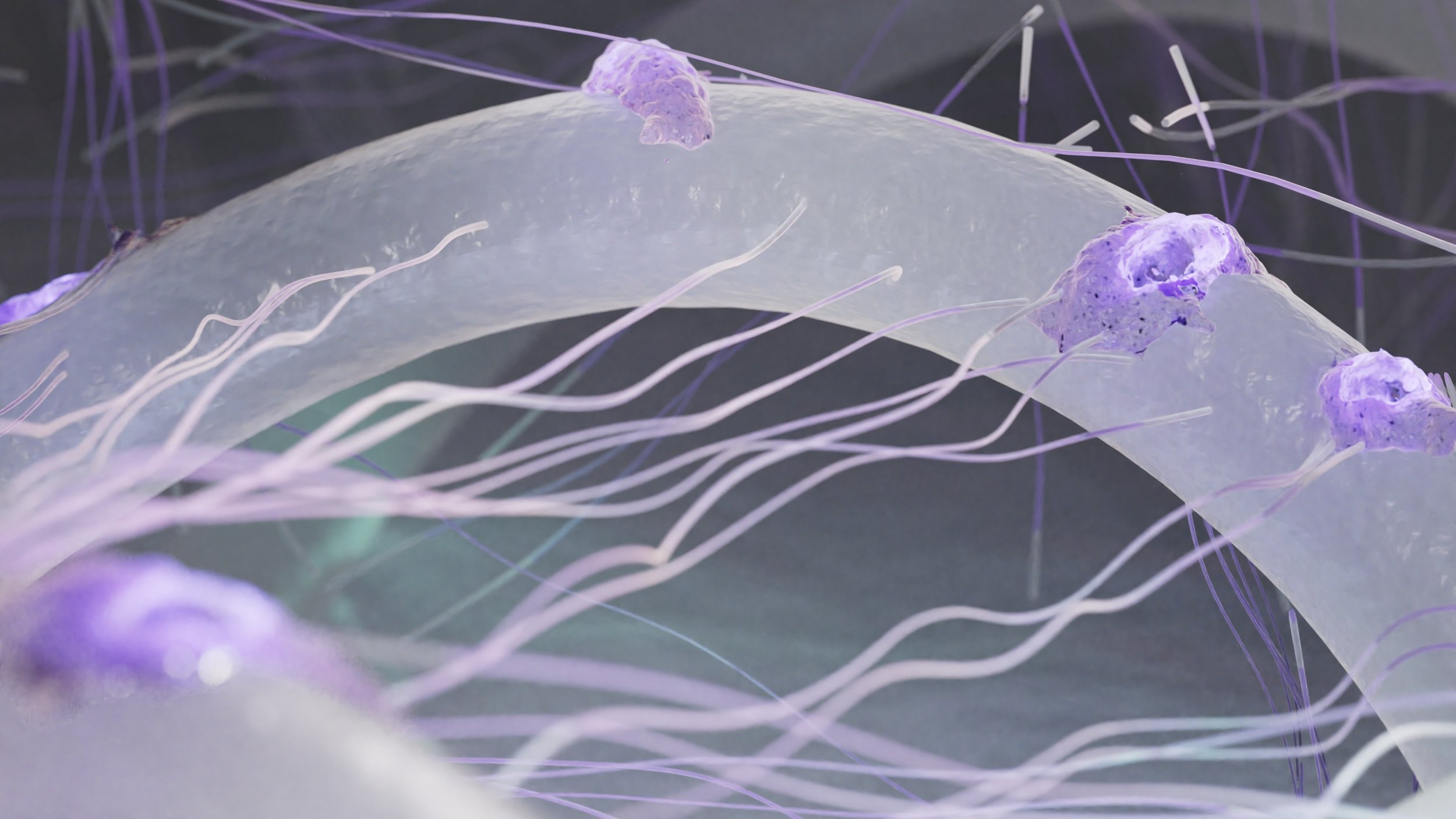
A Biosynthetic Matrix (BSM) Technology
Epidermal Analogue
-
Enabling oxygen, carbon dioxide and exudate to diffuse to/from the wound as needed
Variable slits: Enhance fluid management
Transparent: Visual
Highly flexible: Compliant with anatomy
Hydrophilic: Sustains optimal healing environment
Dermal Analogue
-
Biomimetic fiber 3D micro-support system: Embedded in silicone membrane to stabilize structure throughout the healing process
-
Biological components are added to coating to increase hydrophilic and hygroscopic properties enabling a moist, but not wet environment conducive to healing
-
Structure permits cell migration. Dermis layer enlarges contact area and helps stimulate proliferation and ADHERENCE of cells
Enhanced Clinical Outcome
Improved healing environment
Effective wound closure
Diminished pain
Lower scar formation*
* Clinical Study Data on File
Innovative Product PermeaDerm®
Ultrathin
Transparent
Excellent physical attributes (flexibility, compliance, strength, stretchability, stability)
Easy to secure
Excellent shelf life
Improved User Experience
Effective adherence
Proliferation acceleration
Wound barrier & nerve protection
Single application (superficial wounds)
Visualization of wound healing
Mode of Action - R.A.S.E
Reconstruction of Skin Barrier & ECM
Prevents contamination and protects inner vessels & nerves. Monitors and maintains moisture balance. Provides extracellular matrix environment.
Primary Adherence
Analogue ECM (bio-components) achieved within 5hrs. allowing interaction with fibrin to form a clot within the micro-support system.
Secondary Adherence
3D structure achieved within 72 hours facilitating cell migration, proliferation, and ingrowth.
Epithelialization
Re-epithelialization was achieved, and the wound healed.
Indications
PermeaDerm® Gloves
PermeaDerm®
Partial thickness burns wounds and other partial thickness wounds
Donor sites
coverage of meshed autograft
-
Pressure sores
Venous ulcers
Diabetic ulcers
Vascular ulcers
-
Post-Mohs
Post-laser surgery
Podiatric
Wound dehiscence
-
Abrasions
Lacerations
Skin tears
Draining wounds
Debrided partial thickness hand burns
Case 1: Deep Partial-thickness Burn (Lower Extremity)
Day 0
Day 8
Day 15, healed
6 mos
Case 2: Deep Partial-thickness Hand Burn
Day 0
Day 0, applicaiton
Week 1, healed
Week 3
Week 7